[ad_1]
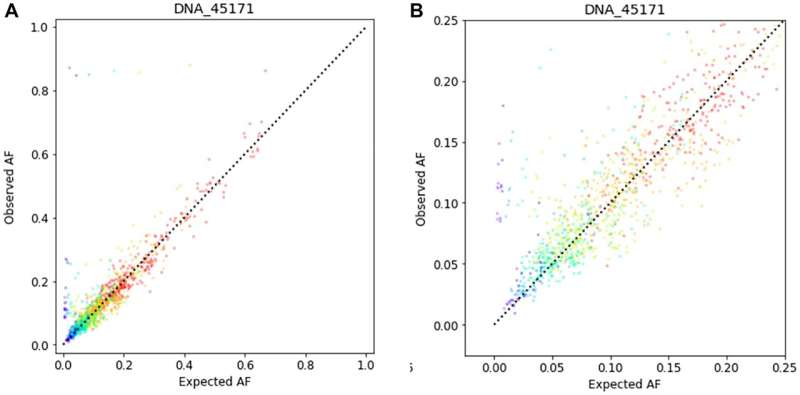
Correlation between noticed AF and anticipated AF for 401 cancer-associated genes in a tumor tissue pattern diluted with the corresponding regular specimen. Credit score: Oncotarget (2023). DOI: 10.18632/oncotarget.28490
A brand new analysis paper titled “Analytic validation of NeXT Dx, a complete genomic profiling assay” has been revealed in Oncotarget.
On this new analysis paper, researchers Juan-Sebastian Saldivar, Jason Harris, Erin Ayash, Manqing Hong, Prateek Tandon, Saloni Sinha, Patricia Miranda Hebron, Erin E. Houghton, Kaleigh Thorne, Laurie J. Goodman, Conan Li, Twinkal R. Marfatia, Joshua Anderson, Massimo Morra, John Lyle, Gabor Bartha, and Richard Chen from Personalis, Inc. describe the analytic validation of NeXT Dx, a complete genomic profiling assay to help remedy and medical trial choice for patients recognized with stable tumor cancers.
“The NeXT Dx medical report at the moment offers the ordering clinician with data from 401 cancer-related genes on clinically related mutations, in addition to associated drug response associations and a curated listing of medical trials which may be relevant to the affected person,” the researchers write.
Proprietary strategies had been utilized to carry out entire exome and entire transcriptome sequencing for detection of single nucleotide variants (SNVs), insertions/deletions (indels), copy quantity alterations (CNAs), and gene fusions, and willpower of tumor mutation burden and microsatellite instability. Variant calling is enhanced by sequencing a patient-specific regular pattern from, for instance, a blood specimen. This offers extremely correct somatic variant calls in addition to the incidental reporting of pathogenic and sure pathogenic germline alterations. Fusion detection through RNA sequencing offers extra intensive and correct fusion calling in comparison with DNA-based exams.
NeXT Dx options the proprietary Accuracy and Content material Enhanced expertise, developed to optimize sequencing and supply extra uniform protection throughout the exome. The exome was validated at a median sequencing depth of >500x. Whereas variants from 401 cancer-associated genes are at the moment reported from the assay, the exome/transcriptome assay is broadly validated to allow reporting of further variants as they grow to be clinically related. NeXT Dx demonstrated analytic sensitivities as follows: SNVs (99.4%), indels (98.2%), CNAs (98.0%), and fusions (95.8%). The general analytic specificity was >99.0%.
The researchers clarify, “By extra comprehensively characterizing the molecular traits of every affected person’s tumor, NeXT Dx offers customized suggestions vital to medical decision-making with respect to present FDA-approved drug-variant particular therapies and evolving therapy alternatives through enrollment in clinical trials.”
Extra data:
Juan-Sebastian Saldivar et al, Analytic validation of NeXT Dx™, a complete genomic profiling assay, Oncotarget (2023). DOI: 10.18632/oncotarget.28490
Supplied by
Impression Journals LLC
Quotation:
Validation of a complete genomic profiling assay (2023, August 30)
retrieved 31 August 2023
from https://medicalxpress.com/information/2023-08-validation-comprehensive-genomic-profiling-assay.html
This doc is topic to copyright. Other than any truthful dealing for the aim of personal research or analysis, no
half could also be reproduced with out the written permission. The content material is offered for data functions solely.
[ad_2]
Source link




Discussion about this post