[ad_1]
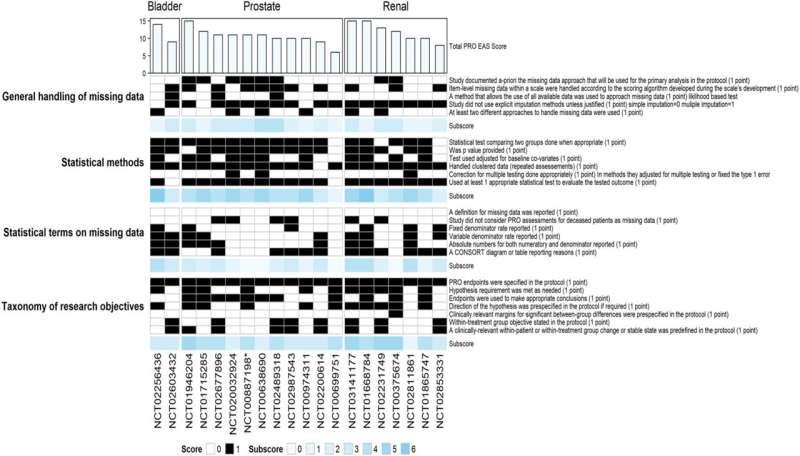
PROEAS graph reporting the completely different PROEAS scores per class for randomized scientific trials individualized by GU tumor sort. Abbreviations: PROEAS: Affected person Reported Final result Endpoint Evaluation Rating; GU: genitourinary. Credit score: eClinicalMedicine (2024). DOI: 10.1016/j.eclinm.2023.102413
Affected person-reported outcomes are essential indicators of how most cancers medicine affect sufferers’ lives. By assessing the advantages and dangers of medicine from a affected person’s perspective, scientists and physicians can enhance the event of patient-centered medicine and care.
In a recent study revealed in eClinicalMedicineMoffitt Most cancers Heart researchers reveal that there’s a important unmet want for improved analyses and reporting of patient-reported outcomes in genitourinary most cancers clinical trials.
Well being care professionals have realized that how a illness and its remedy affect a affected person’s life are vital components to think about when selecting remedy. A remedy could enhance survival; nonetheless, if its unwanted effects are too nice and affect a affected person’s high quality of life, that drug will not be an optimum selection.
Affected person-reported outcomes are experiences from sufferers about their very own well being standing and high quality of life that consider their illness, the remedy they’re receiving, and different private components. Affected person-reported outcomes can embrace questions from unwanted effects of remedy to monetary issues and entry to caregiver assist.
The Moffitt researchers needed to evaluate the standard of patient-reported outcomes information collected in scientific trials that led to the approval of genitourinary most cancers therapies. Genitourinary cancers have an effect on over 444,000 folks annually in the USA. They’re among the many commonest malignant illnesses in males, together with prostate, bladder and kidney cancers.
Chemotherapy has historically been the usual remedy choice for sufferers with genitourinary cancers; nonetheless, throughout the previous 15 years, many new focused treatment options have been accepted primarily based on enhancements in outcomes equivalent to total survival and response charges. Affected person-reported outcomes are sometimes analyzed in most cancers scientific trials, however the high quality of those analyses in genitourinary scientific trials is unknown.
The workforce performed a scientific information evaluation that led to genitourinary most cancers drug FDA approvals from February 2007 to July 2022 and recognized 40 scientific trials that met inclusion standards. They analyzed the scientific trial protocols and in contrast what was supposed within the research to what patient-reported outcomes information was offered and revealed. The researchers then analyzed the standard of the patient-reported outcomes information primarily based on a scoring scheme they developed.
The researchers discovered that solely 67.5% of the trial publications reported patient-reported outcomes information, together with 10% that reported preliminary patient-reported outcomes information within the major scientific trial publication and 57.5% that reported patient-reported outcomes information in a secondary publication. Of the 40 scientific trials, 31 deliberate to gather patient-reported outcomes information, whereas 9 didn’t.
The median time between the first scientific trial publication and the publication of the patient-reported end result was 10.5 months. The researchers discovered that the kind of patient-reported outcomes information that was collected and the statistical analyses used to investigate the info diverse significantly among the many research, and plenty of trials nonetheless wanted to carry out a top quality evaluation. The imply high quality rating of the patient-reported outcomes information was 11.10 (vary of 6–24) out of a attainable 24 complete factors.
These mixed information reveal that patient-reported outcomes information is usually not collected in a well timed method or of the very best high quality. The researchers hope their evaluation will enhance scientific trial design and approaches to amassing and analyzing patient-reported outcomes information to enhance affected person outcomes and high quality of life.
“Our information confirmed a substantial hole in reporting patient-reported outcomes and within the high quality of design and conduct of patient-reported outcomes-related trial endpoints. With the increasing array of therapies for genitourinary malignancies, the accessibility and clear presentation of patient-reported outcomes information are additionally important for tailoring customized remedy plans for sufferers, highlighting the essential want for improved reporting and accessibility of this data,” defined Jad Chahoud, M.D., MPH, assistant member of the Division of Genitourinary Oncology at Moffitt.
Extra data:
Mahati Paravathaneni et al, 15 years of patient-reported outcomes in scientific trials resulting in GU most cancers drug approvals: a scientific evaluation on the standard of knowledge reporting and evaluation, eClinicalMedicine (2024). DOI: 10.1016/j.eclinm.2023.102413
Quotation:
Examine suggests enhancements wanted for patient-reported end result information in genitourinary most cancers scientific research (2024, February 6)
retrieved 6 February 2024
from https://medicalxpress.com/information/2024-02-patient-outcome-genitourinary-cancer-clinical.html
This doc is topic to copyright. Other than any truthful dealing for the aim of personal research or analysis, no
half could also be reproduced with out the written permission. The content material is supplied for data functions solely.
[ad_2]
Source link




Discussion about this post