[ad_1]
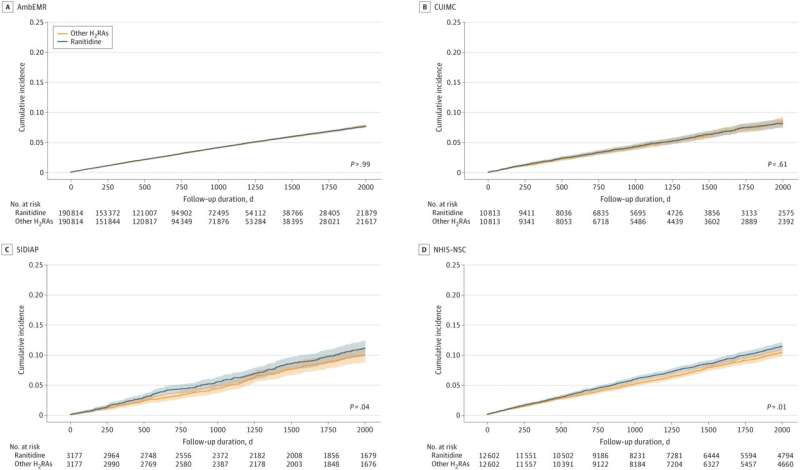
Kaplan-Meier Plots for the Threat of All Most cancers Besides Nonmelanoma Pores and skin Most cancers Related With Ranitidine Customers and Different Histamine-2 Receptor Antagonist (H2RA) Customers P-values in survival curves had been estimated utilizing Cox proportional hazard regression fashions. Shading within the survival curves signifies 95% CIs. AmbEMR signifies IQVIA US Ambulatory Digital Medical Analysis; CUIMC, Columbia College Irving Medical Middle knowledge warehouse; NHIS-NSC, Korean Nationwide Well being Insurance coverage System-Nationwide Pattern Cohort; SIDIAP, The Data System for Analysis in Main Care. Credit score: JAMA Community Open (2023). DOI: 10.1001/jamanetworkopen.2023.33495
Analysis led by Seng Chan You, MD, on the Yonsei College School of Drugs, Korea, has investigated the danger of most cancers related to use of the drug ranitidine in comparison with different histamine-2 receptor antagonists (H2RAs) utilizing a large-scale cohort examine throughout a number of nations.
Within the paper, “Ranitidine Use and Incident Most cancers in a Multinational Cohort,” printed in JAMA Community Openthe researchers declare that regardless of contamination with a possible human carcinogen N-nitrosodimethylamine (NDMA) present in ranitidine, there was no statistically vital proof that publicity to the drug was related to an elevated danger of most cancers.
In a outcomes assertion the authors declare, “On this cohort study together with 1,183,999 people from 11 giant databases throughout Europe, North America, and Asia, danger of most cancers amongst ranitidine customers didn’t differ from customers of different H2RAs. Ranitidine use was not related to an elevated danger of esophageal, abdomen, or colorectal most cancers, or 13 different subtypes of most cancers.”
The information offered of their examine refutes this consequence assertion in some vital methods. First, the precise cohort was a lot smaller.
To check ranitidine use to options accessible inside cohorts at a 1:1 ratio, the precise knowledge used for the examine’s major evaluation included 434,812 people from 4 databases, half of which had been uncovered to ranitidine.
One in every of these databases, IQVIA US Ambulatory Digital Medical Analysis (AmbEMR), accounts for 381,628 people, due to this fact making up the majority of the first evaluation and has the shortest follow-up interval of ranitidine customers at 2.61 years.
Additionally included within the major evaluation had been the Columbia College Irving Medical Middle knowledge warehouse (CUIMC) databases (imply follow-up of three.6 years), NHIS-NSC, Korean Nationwide Well being Insurance coverage System-Nationwide Pattern Cohort (NHIS-NSC) with a imply follow-up of 4.5 years, and Spain’s Data System for Analysis in Main Care (SIDIAP) with a imply 5.8-year period follow-up.
A subgroup meta evaluation was supplied with the excluded cohorts and the numbers had been lowered to match the decrease charges of native use of options to ranitidine. Within the case of the U.Ok.’s IQVIA Medical Analysis Information (IMRD), the variety of ranitidine customers was lowered from 178,298 to simply 633 people.
Whereas the hazard ratio (HR) throughout 16 completely different cancers within the major examine of 4 cohorts over 4 completely different follow-up durations was 1.04 general, some particular person most cancers varieties confirmed extra vital HR.
The authors acknowledged that ranitidine use was not related to an elevated danger of esophageal (HR 1.08) or abdomen (HR 1.17) cancers, although 8% and 17% elevated occasions appear vital.
The authors acknowledged that ranitidine was not related to elevated danger in 13 different cancers like leukemia (HR 1.12) and gallbladder/ biliary tract (HR1.14) most cancers, although 12% and 14% will increase seem vital.
It’s unclear how corpus uteri endometrial (HR 1.20) and Ovary (HR 1.26) most cancers charges weren’t thought-about above the edge of serious occasion associations, particularly in such a short-term examine.
It could be fascinating to know the age ranges of the ladies with these occasions as NDMA can intrude with DNA replication throughout cell division, one thing extra prone to be occurring in reproductive age ovary and corpus uteri tissues, however that knowledge was excluded from the examine.
Exposures to carcinogens are recognized to contribute to later-life illness formation even after the publicity supply is eliminated, requiring long run examine, which is why the drug was pulled off the market within the first place.
Ranitidine and NDMA
Ranitidine, bought underneath the model title Zantac, was as soon as extensively used to deal with circumstances like heartburn and peptic ulcer illness however was withdrawn from the market in 2020 by request of the FDA as a consequence of contamination with NDMA. The FDA investigation discovered elements like warmth and the period of time since ranitidine was manufactured led to larger ranges of NDMA. These circumstances might elevate the extent of NDMA within the ranitidine product above the appropriate every day consumption restrict.
NDMA is a DNA-damaging agent that creates 3-methyladenine, which might intrude with the copying of DNA. Broken DNA causes mutations that drive most cancers, which can take a few years to develop.
Publicity to NDMA has been particularly linked to childhood most cancers charges in a real-world state of affairs. The Olin Company chemical manufacturing plant, now a chemical superfund web site, in Wilmington, MA, uncovered native residents to excessive ranges of NDMA by means of drinking water for a number of years. The publicity was solely found by means of the weird cluster of twenty-two kids in a city with lower than 22,000 individuals who bought most cancers. The ingesting water was ultimately examined, and NDMA was recognized in a uncommon case the place isolating a single kind of extended chemical publicity might be linked to well being outcomes.
MIT researchers adopted up with research on mice and decided that the kid most cancers charges had been possible initiated in utero when cell division was highest. In addition they discovered an fascinating pivot level for the consequences of NDMA that had been depending on the degrees of Alkyladenine DNA glycosylase (AAG) enzymes.
An excessive amount of cell division ends in most cancers when AAG is low versus cell demise when AAG is excessive. People can range by as a lot as 10-fold of their ranges of AAG, requiring any examine of NDMA results to incorporate alerts of degenerative illness, one thing lacking from the present examine.
Who cares a few drug that’s off the market?
First accepted for U.S. markets in 1983, ranitidine (Zantac) was one of many first drugs to achieve $1 billion in gross sales and went on to generate $4 billion in yearly gross sales earlier than being pulled from the market. A number of drug companies have bought both the prescription, over-the-counter, branded or generic model of the drug earlier than the FDA suggested shoppers to eliminate any ranitidine merchandise. The recall broken a number of firm reputations, inventory values they usually have been combating litigation and legal responsibility points ever since.
The model Zantac has been reformulated as Zantac 360º OTC and now incorporates famotidine, one of many FDA accepted non NDMA forming H2RAs ranitidine was in contrast in opposition to within the present examine.
It’s unclear why the researchers selected to check ranitidine, a drug that’s at present off the market and unlikely to return. In an announcement in regards to the limitations of the examine the authors state, “The examine interval was comparatively quick and should underestimate the most cancers danger related to the longer-term use of ranitidine,” and but are nonetheless in a position to conclude that their findings “…don’t assist proactive cancer screening or surveillance amongst people beforehand uncovered to ranitidine.”
Within the battle of curiosity disclosures part, lead writer Dr. Seng Chan You reported receiving private charges from IQVIA, who curates the principle databases used within the examine. IQVIA additionally has an worker listed as an writer of the paper who was concerned in design, evaluation, interpretation of knowledge, and supervision of the examine.
Receiving private charges from IQVIA and having an IQVIA worker concerned within the analysis shouldn’t be unusual in some pharmaceutical analysis circles. IQVIA is a number one world supplier of superior analytics, know-how options and scientific analysis providers to the life sciences business and participation on some degree is likely to be anticipated, although it’s value noting that the worker concerned within the present examine has a powerful “technique” focus inside IQVIA.
IQVIA additionally has a significant model advertising and promotion aspect to the corporate and their web site incorporates typical examples of selling technique lingo that in most industrial contexts can be anticipated, however might be inappropriate in a analysis setting like “Leverage proof to drive affected person entry at a value that maximizes worth for well being care stakeholders.”
As with all analysis, transparency in potential conflicts of curiosity is crucial. With the multifaceted ways in which an organization like IQVIA might be conflicted, a battle of curiosity disclosure from them appears warranted on any examine they’re concerned with.
Extra info:
Seng Chan You et al, Ranitidine Use and Incident Most cancers in a Multinational Cohort, JAMA Community Open (2023). DOI: 10.1001/jamanetworkopen.2023.33495
Bevin P. Engelward, Implications of an epidemiological examine displaying an affiliation between in utero NDMA publicity and childhood most cancers, Environmental and Molecular Mutagenesis (2021). DOI: 10.1002/em.22434
© 2023 Science X Community
Quotation:
Ranitidine examine finds no elevated danger of most cancers… regardless of discovering elevated most cancers charges (2023, September 22)
retrieved 23 September 2023
from https://medicalxpress.com/information/2023-09-ranitidine-elevated-cancer.html
This doc is topic to copyright. Other than any truthful dealing for the aim of personal examine or analysis, no
half could also be reproduced with out the written permission. The content material is offered for info functions solely.
[ad_2]
Source link




Discussion about this post