[ad_1]
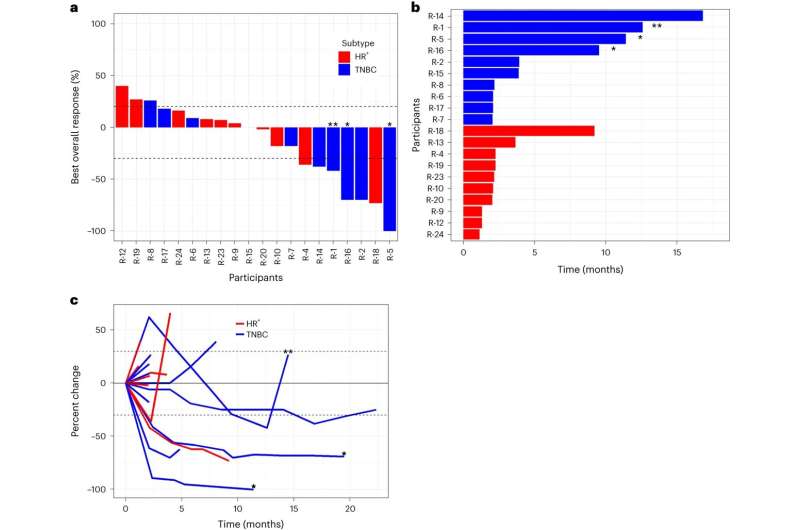
identifies contributors with continued profit past information cutoff. Double asterisks (**) determine contributors with response after remedy past development; N = 20 contributors. Credit score: Nature Most cancers (2024). DOI: 10.1038/s43018-024-00729-w Scientific efficacy.a–cResponses as proven by a waterfall plot (a), swimmers plot (b) and spider plot (c ). The dashed strains correspond to +20% and –30%, respectively. The only asterisk identifies contributors with continued profit past information cutoff. Double asterisks (**) determine contributors with response after remedy past development; N = 20 contributors. Credit score:
Nature Most cancers
(2024). DOI: 10.1038/s43018-024-00729-w
A novel three-drug mixture achieved notable responses in sufferers with superior HER2-negative breast most cancers, in keeping with new analysis directed by investigators from the Johns Hopkins Kimmel Most cancers Heart. The treatment included a histone deacetylase (HDAC) inhibitor—a drug that causes a chemical change to stop tumor cells from dividing—with two types of immunotherapy known as checkpoint inhibitors, which unharness the power of the immune response against cancer.The multicenter part IB examine, which aimed to enhance response to checkpoint inhibitors by sensitizing the tumor microenvironment discovered that the
mixture remedy resulted in a 25% overall response rate (ORR) in women with advanced HER2 (human epidermal growth factor receptor 2)-negative breast cancer.Because of this for 25% of girls who obtained the remedy, their most cancers was destroyed or considerably decreased. Sufferers with triple-negative breast cancer who’ve fewer remedy choices than sufferers with different breast cancers, had an ORR of 40%. These outcomes had been printedin
Nature Most cancers .“Our findings present that pre-treatment with the HDAC inhibitor entinostat and using twin immune checkpoint inhibitors is a secure and promising technique for metastatic breast cancer warranting additional
medical analysis
in a part II examine,” says lead creator Evanthia Roussos Torres, M.D., Ph.D., an assistant professor on the College of Southern California Keck Faculty of Drugs who was on the Johns Hopkins College Faculty of Drugs and Kimmel Most cancers Heart when the work was carried out.
“These outcomes actually met our speculation that we might enhance response to checkpoint inhibition in metastatic breast most cancers.”
This examine was carried out throughout 4 medical websites and included 24 ladies with HER2-negative metastatic breast most cancers. Twelve had hormone receptor-positive illness, and 12 had the triple-negative illness. All obtained entinostat plus two forms of immunotherapy: the PD-1/PD-L1 inhibitor nivolumab and the CTLA-4 inhibitor ipilimumab.
The examine met its main endpoint of security by demonstrating that there have been anticipated and tolerable toxicities amongst sufferers, with none requiring discontinuation of remedy. The typical progression-free survival (PFS) was 50% at six months, which means for half of the contributors, their illness didn’t worsen for at the very least six months. Future research will handle the remedy’s efficacy. “To our knowledge, this is the first published study that investigates treatment with an HDAC inhibitor in combination with dual immune checkpoint inhibitor therapy in patients with advanced breast cancer,” says co-author Elizabeth Jaffee, M.D., the Dana and Albert “Cubby” Broccoli Professor of Oncology and deputy director of the Johns Hopkins Kimmel Cancer Center. “Closely pre-treated
superior breast most cancers remains an area of unmet need, and a combination strategy that results in the ORR and PFS described is of interest.”For a lot of sufferers with
strong tumors
immune checkpoint inhibitors have change into cornerstones of remedy by releasing the inhibition operate of varied forms of immune cells and permitting sufferers’ immune methods to assault most cancers cells. This technique has up to now been ineffective for many breast cancers.
Conversely, epigenetic medicine that silence the genes concerned in most cancers growth have been studied with single immune checkpoint inhibitors for breast most cancers to alter the immune response within the tumor microenvironment. Twin immune checkpoint inhibitors have been proven to be secure when utilized in mixture for a wide range of cancers.
“Though we did see superb responses, the query is whether or not we did it by altering the tumor microenvironment, however the examine was not powered to reply this query,” Roussos Torres says. “Our examine highlights the necessity for a deeper investigation of the breast most cancers tumor microenvironment with a concentrate on modifications in myeloid (immune) cell populations to find out their position in sensitization of the tumor microenvironment to remedy with immune checkpoint inhibitors.”
“This medical trial highlights the significance of interdisciplinary collaboration to carry an thrilling speculation from the laboratory to the clinic, and units the scene for future trials investigating novel remedy approaches for this affected person inhabitants,” says Roisin Connolly, M.D., M.B.B.Ch., who was lead principal investigator for the trial alongside Vered Stearns, M.D. Connolly is now on the College School Cork in Eire. Stearns is now at Weill Cornell Drugs in New York. About 300,000 people in the United States receive a breast cancer diagnosis each year, making it the most common cancer among American women apart from skin cancers. Of these, about 70% are hormone receptor-positive and HER2-negative, meaning the tumor cells contain receptors for estrogen or progesterone but low levels of the protein HER2. Solely about 10% to fifteen% of breast most cancers instances are what’s generally known as triple adverse, which means
tumor cells
don’t have any hormone receptors and low HER2 ranges, making them tougher to deal with than different forms of breast most cancers. Extra data: Evanthia.T. Roussos Torres et al, Entinostat, nivolumab and ipilimumab for girls with superior HER2-negative breast most cancers: a part Ib trial, Nature Cancer
(2024).
DOI: 10.1038/s43018-024-00729-w
Supplied byJohns Hopkins College Faculty of Drugs
Quotation
:
Novel drug mixture reveals promise for superior HER2-negative breast most cancers (2024, February 14)
retrieved 19 February 2024
from https://medicalxpress.com/information/2024-02-drug-combination-advanced-her2-negative.htmlThis doc is topic to copyright. Other than any truthful dealing for the aim of personal examine or analysis, no
half could also be reproduced with out the written permission. The content material is offered for data functions solely.
[ad_2]
Source link




Discussion about this post