[ad_1]
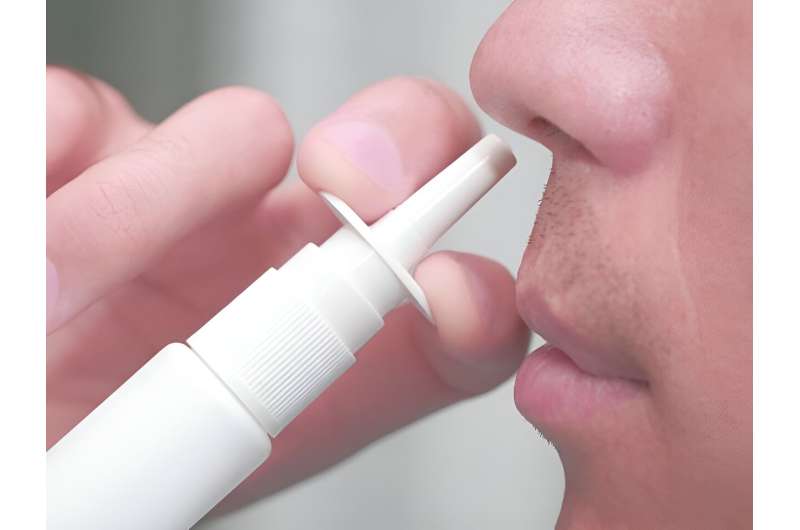
New analysis factors to the potential of a COVID-19 vaccine delivered by means of the nostril.
The section 1 medical trial confirmed that the product, administered nasally in two doses, delivered a major immune response to a number of COVID variants.
Known as CoviLiv, the vaccine was examined as a main vaccination collection on healthy adults earlier than growth of the mRNA vaccines that are actually permitted to deal with COVID.
As a substitute, CoviLiv is a live-attenuated vaccine, which means it’s comprised of weakened virus. Genetic materials of the virus was recoded to transform it from a disease-causing pathogen right into a secure and secure vaccine, in keeping with its developer, Codagenix Inc.
Members who acquired the vaccine throughout the trial had sturdy immune responses, in keeping with an organization information launch in regards to the research. Additionally they had T-cell reactivity that was seen to be particular for a number of viral antigens past the incessantly mutating coronavirus spike protein.
The thought was to provide an immune response to all the virus fairly than the incessantly mutating spike protein. This might probably present broader safety in opposition to variants, the researchers stated.
None of the present COVID vaccines are live-attenuated or delivered nasally.
The findings are to be offered Wednesday at IDWeek 2023, the joint annual assembly of a number of organizations, together with the Infectious Illness Society of America, in Boston.
“The research findings present a glimpse into what may very well be the subsequent technology of COVID-19 vaccines that present differentiated safety to extra individuals,” stated lead research writer Johanna Kaufmann, govt vp for oncology and immunology at Codagenix Inc.
“Vaccine administration by nostril and simpler storage can enhance entry to vaccinations for underserved areas internationally,” she stated in a gathering information launch.
CoviLiv doesn’t require chilly chain storage, which might make it simpler to stockpile in areas that do not have sufficient refrigeration. Having an alternative choice to COVID pictures may enhance uptake in areas with decrease vaccination charges, in keeping with the research.
Findings offered at medical conferences are thought of preliminary till revealed in a peer-reviewed journal.
Extra info:
The U.S. Facilities for Illness Management and Prevention has extra on COVID-19 vaccines.
Copyright © 2023 HealthDay. All rights reserved.
Quotation:
Nasal spray COVID vaccine reveals promise in early trial (2023, October 11)
retrieved 11 October 2023
from https://medicalxpress.com/information/2023-10-nasal-spray-covid-vaccine-early.html
This doc is topic to copyright. Other than any honest dealing for the aim of personal research or analysis, no
half could also be reproduced with out the written permission. The content material is offered for info functions solely.
[ad_2]
Source link




Discussion about this post