[ad_1]
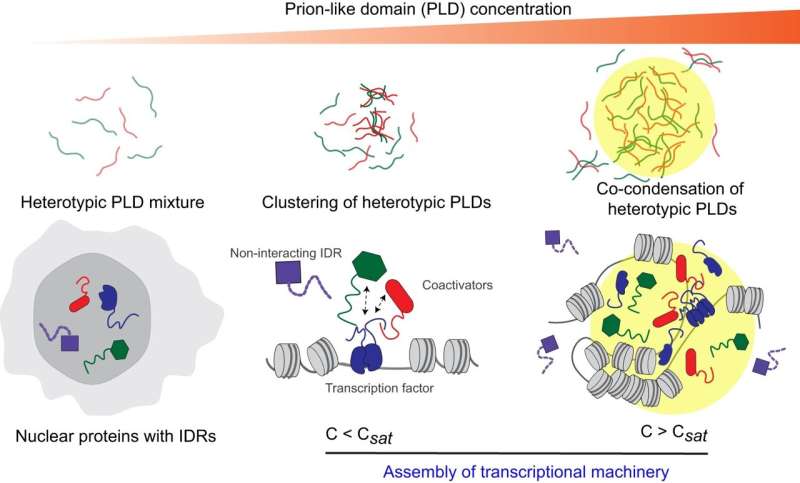
Schematic illustration of heterotypic PLD-mediated co-assemblies driving practical protein interplay networks. Heterotypic PLD interactions happen at sub-saturation concentrations which upon rising protein focus can result in co-phase separation of the combination into spatially homogeneous multi-component condensates (prime panel). Our outcomes collectively recommend that transcriptional proteins may be assembled into co-phase-separated hubs via sequence-specific positively cooperative interactions amongst low-complexity domains (backside panel). Credit score: Nature Communications (2024). DOI: 10.1038/s41467-024-44945-5
Many childhood cancers begin with a hijacking on the molecular stage. A bunch of irregular proteins referred to as fusion proteins aberrantly engages with a group of proteins that switches genes on and off. Consequently, genes that ought to be activated get repressed, and genes that ought to be repressed get activated, inflicting most cancers.
College at Buffalo researchers have now shed extra mild on the molecular mechanism behind this hijacking. Their examine, printed in Nature Communications, exhibits that the fusion proteins and a gene regulatory protein complex work together via their unfolded, floppy areas, referred to as disordered domains.
Regardless of their fuzzy nature, the disordered domains work together with a excessive diploma of specificity by forming liquid-like droplets and mixing them collectively.
“There was a whole lot of proof that disordered areas of proteins play a job in most cancers and different main human ailments, however the query has been how,” says the examine’s senior corresponding writer, Priya Banerjee, Ph.D., affiliate professor within the Division of Physics, throughout the UB Faculty of Arts and Sciences.
“Right here we present that this significant networking occasion between the fusion proteins and the gene regulatory protein advanced occurs via their disordered domains and that there’s a chemical specificity to this course of.”
Fusion proteins are the results of a chunk of chromosome breaking off and attaching to a different to type a fusion gene. Fusion proteins do not all the time result in most cancers, however some can alter the exercise of different genes to the purpose the place cells develop uncontrollably and trigger most cancers. These are referred to as fusion oncoproteins.
“To ensure that most cancers to develop, so much must go mistaken. That’s, a whole lot of gene mutation must occur. However within the case of some pediatric cancers, these fusion oncoproteins could be the only offender,” says the examine’s lead writer, Richoo Davis, Ph.D., a postdoc in Banerjee’s lab. “So focusing on oncoproteins alone could be adequate sufficient to treatment such childhood cancers.”
Breakthrough research by different teams have proven that fusion oncoproteins alter gene exercise by hijacking a significant chromatin remodeler referred to as the mammalian SWI/SNF advanced. Nearly 20% of all human cancers have a mutation on this protein advanced.
Banerjee’s workforce needed to understand how precisely fusion oncoproteins hijack the advanced. Their new paper, which builds on their previous study published in 2021finds that each the fusion oncoproteins and the mammalian SWI/SNF advanced work together via their respective disordered domains.
Unable to fold into distinctive three-dimensional shapes, dysfunction domains have lengthy remained enigmatic. Scientific dogma for many years was that protein interactions occur by way of proteins’ structured areas coming collectively in a lock-and-key-like mechanism.
Nevertheless, analysis within the final twenty years has proven that nearly 30% of the human proteome is disordered and that dysfunction areas drive some key organic processes and main human ailments.
Banerjee’s workforce checked out a particular disordered domainreferred to as prion-like domains, from fusion oncoproteins and the gene regulatory advanced and located these explicit domains type liquid-like droplets, or membrane-less organelles, within the nucleus of the cell.
Molecular complexes type from the prion-like domains of each the fusion oncoproteins and the gene regulators when their networking begins. These complexes then mix to type liquid-like droplets or co-condensates. To review this additional, Davis used a blue light-activated droplet-forming system that allowed the workforce to view these processes extra clearly. Nevertheless, there’s beautiful specificity of what can co-condense collectively to activate aberrant gene expression resulting in most cancers.
“We discovered that disordered areas of repressor proteins do not actually enrich within the droplets fashioned by prion-like domains, and we predict that this selectivity in molecular networking is a vital mechanism by which fusion oncoproteins contributes to most cancers improvement,” Banerjee says.
As soon as the fusion proteins have efficiently hijacked the advanced, they primarily can take it away from the genes it is presupposed to activate—and produce it to genes it mustn’t activate.
Sooner or later, pharmaceutical drugs may very well be developed to focus on the irregular networking of fusion proteins with the SWI/SNF advanced as a way to treatment most cancers.
“This elementary analysis is essential,” Banerjee says. “We can not treatment a deadly human illness until we absolutely perceive the molecular wiring beneath it.”
Extra data:
Richoo B. Davis et al, Heterotypic interactions can drive selective co-condensation of prion-like low-complexity domains of FET proteins and mammalian SWI/SNF advanced, Nature Communications (2024). DOI: 10.1038/s41467-024-44945-5
Supplied by
University at Buffalo
Quotation:
How fusion proteins hijack gene regulators to spur childhood most cancers (2024, March 6)
retrieved 10 March 2024
from https://medicalxpress.com/information/2024-03-fusion-proteins-hijack-gene-spur.html
This doc is topic to copyright. Aside from any honest dealing for the aim of personal examine or analysis, no
half could also be reproduced with out the written permission. The content material is supplied for data functions solely.
[ad_2]
Source link




Discussion about this post