[ad_1]
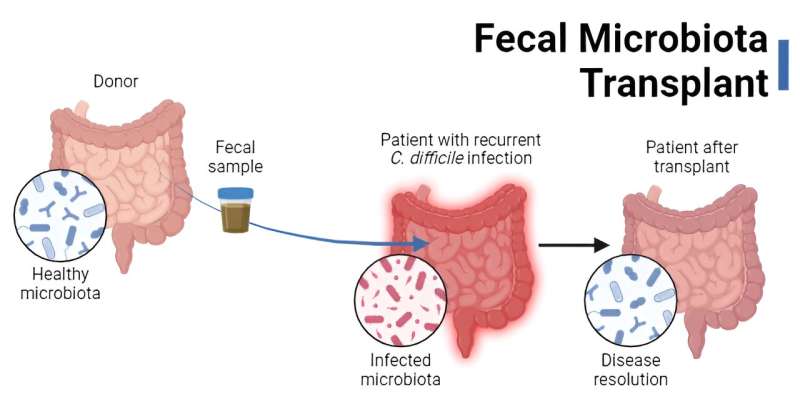
The premise of fecal microbiota transplants (FMT) is, admittedly, not essentially the most nice. The method entails transferring donor stool (or derivatives thereof) to a recipient for a therapeutic objective—specifically, to revive the microbiota to a state able to resisting the intestine pathogen Clostridioides difficile.
However the place did the thought for FMTs even come from? What’s new within the discipline of transferring feces—and what does the longer term maintain?
It started with feces soup
The beginning of FMTs precedes data of microbes, a lot much less the gut microbiota. The primary information date again to 4th century China, the place “yellow soup” (i.e., human fecal slurry) was used to deal with sufferers with extreme diarrhea and meals poisoning. Although fairly probably the worst soup ever, it was reported to have “brought patients back from the brink of death.”
Quick ahead to 1958, when the primary “fashionable” FMT was carried out. On this case, fecal enemas were used to cure 4 patients with pseudomembranous colitis, possible attributable to C. difficile. Usually, the gut microbiota resists colonization by C. difficile. Nevertheless, if the microbiota is disrupted, sometimes following antibiotic treatment for one more an infection, the pathogen can survive and thrive—secreting toxins, inflicting diarrhea and damaging the gut.
The researchers within the 1958 examine knew that antibiotics presumably killed off intestine microbes and hypothesized that reintroducing “regular” micro organism into the intestine by way of the fecal enemas would “re-establish the balance of nature.” The observe wasn’t intentionally used for C. difficile an infection (CDI) until the 1980s, with some success.
But, the therapy by no means took off. Krishna Rao, M.D., M.S., the co-founder and director of the Fecal Microbiota Transplantation program on the College of Michigan, factors to a few explanation why. For one, there was the “ick” issue—the thought of administering a slurry of feces to individuals by way of enema wasn’t very interesting to sufferers or practitioners.
Second, “CDI was a a lot totally different illness again then,” he mentioned. “It was seen as a nuisance, not a large risk and burden on the well being care system.” (Today, C. difficile causes nearly half a million infections and 30,000 deaths in the U.S. every year). Whereas antibiotics are the usual therapy for CDI, in some instances they do not work, and the an infection will come again (recur), resulting in a vicious and doubtlessly lethal cycle of illness.
Based on Rao, realization of the complete risk of CDI within the 2000s—and rising charges of recurrent illness—helped convey FMT into the sunshine. However a “landmark” 2013 trial exploring the efficacy of FMT for recurrent CDI gave the observe legs.
The trial confirmed that infusion of donor stool was extremely efficient at treating sufferers with recurrent CDI, in comparison with vancomycin administration alone (94% versus 31% remedy charge, respectively). Actually, it was so efficient that the examine was stopped early, and FMTs have been administered to sufferers in different therapy arms as a result of not doing so was thought of unethical.
Later that yr, the U.S. Meals and Drug Administration (FDA) introduced it could exercise enforcement discretion when FMT was used to deal with recurrent CDI. That’s, sufferers would not need to receive treatment via a medical trial, as can be required if FMTs have been used for different circumstances. This announcement made FMT extra accessible to physicians and, finally, CDI sufferers.
How are FMTs used right now?
Immediately, FMT is commonly reserved for individuals with recurrent CDI who’ve failed 2 rounds of antibiotic therapy or have extreme illness. The process is generally considered safe, and most unwanted effects are transient and localized to the intestine (e.g., bloating, constipation, and so on.). The remedy charge of FMT for recurrent CDI hovers round 80-90% (although evaluation of solely essentially the most rigorous, managed clinical trials suggests it might be closer to 70%).
FMTs are (fortunately) not given to sufferers as a soup nowadays, and enemas are not the only route of administration. FMTs are additionally administered by way of colonoscopy and typically with a nasogastric tube (a tube that goes via the nostril into the abdomen). Donor fecal materials may also be encapsulated into pills and brought orally. No matter how they’re packaged, donor feces are given to sufferers solely after cautious screening for varied pathogens, together with methicillin-resistant Staphylococcus aureus, vancomycin-resistant Enterococcus, HIV, and others.
Feces for FMTs are typically obtained from stool banks, the biggest being OpenBiome, a non-profit group that collects, screens, and shops stool from wholesome donors. Physicians can order fecal materials from OpenBiome (or different banks) to manage to sufferers. For a few years, there have been no industrial merchandise in the marketplace—till now.
The rise of economic FMT merchandise
In Nov. 2022, the FDA permitted the first commercial FMT product, RBX2660 (Rebyota), for prevention of recurrent CDI in adults. Ready from donor stool, every dose is run rectally. Like normal FMT stool suspensions, the exact combination of microbes in RBX2660 is undefined.
Nevertheless, the product does guarantee a minimal focus of some micro organism, akin to Bacteroides, that are members of the “regular” microbiota that assist resist C. difficile colonization. In a part 3 trial, RBX2660 was significantly better at stopping recurrent CDI in contrast with placebo (70.6% success versus 57.5%).
One other development got here in April 2023 with the FDA approval of SER-109 (Vowst)—the primary industrial oral FMT product for recurrent CDI. Not like RBX2660 and different stool preparations, which comprise a hodgepodge of microbes, SER-109 comprises solely donor-derived spores of Firmicutes micro organism, one of many predominant phyla within the microbiota.
Depletion of those micro organism (e.g., due to antibiotic therapy) can create a metabolic environment that promotes CDI recurrence. Replenishing Firmicutes by way of SER-109 is postulated to revive colonization resistance in opposition to the pathogen. Certainly, in sufferers with 3 or extra cases of CDI in 1 yr, SER-109 led to a lower recurrence rate after 8 weeks in comparison with placebo therapy (12.4% versus 39.8%, respectively).
For Rao, the SER-109 product has some additional perks, together with the truth that sufferers can take the drugs at dwelling. “Now, if anybody, regardless of the place they reside within the nation, sees a affected person that wants this therapy, they will write a script, fill out a type, and [the product] will get shipped to their home,” he famous. “That is a whole recreation changer when it comes to availability of this modality.”
The FDA stamp of approval for each RBX2660 and SER-109 additionally has essential logistical ramifications. “The truth that these are actually FDA permitted, and at the least have the hope of getting coated by insurance coverage—one thing that was by no means the case with the OpenBiome product—is a [huge benefit],” Rao mentioned. And these merchandise do not come low-cost; a single dose of OpenBiome costs over $1,600, whereas RBX2660 and SER-109 have sticker prices of $9,000 and $17,500, respectively.
Furthermore, the FDA revised its guidelines in 2022 to state that it could train enforcement discretion just for stool ready in well being care services, not stool banks. This makes buying stool a problem that FDA-approved merchandise could assist overcome.
Although FMTs are actually the usual of look after recurrent CDI, there are nonetheless questions in regards to the observe that may proceed to form its improvement and potential makes use of.
How do FMTs work—and who’re they for?
Regardless of the title, the mechanisms of FMT are considerably of a thriller. The final concept is that FMTs repopulate the gut with microbes able to resisting C. difficile. However which microbes are wanted—and are they wanted in any respect?
The efficacy of SER-109 means that solely the spore-forming fraction of the microbiota is required, but sterile fecal filtrate (i.e., missing microbes) additionally restored normal stool habits and eliminated symptoms in sufferers with CDI. “What we thought was crucial and adequate [for FMTs to work] seems to not be,” Rao mentioned. “That raises the query, “Properly, what are the required and adequate parts of feces with a view to induce a therapeutic response?” And we nonetheless do not know that.”
Figuring out which sufferers ought to obtain a FMT can be unclear. When the COVID-19 pandemic hit, Rao needed to droop College of Michigan’s FMT program for over a yr. Throughout that point, he handled sufferers with antibiotics for longer than regular and leaned into holistic care (e.g., encouraging sufferers to eat fermented meals, which have anti-inflammatory results). The kicker: the sufferers received higher—his remedy charge was north of 90%.
A multi-faceted therapy method that features prolonged programs of antibiotics and weight-reduction plan, amongst different parts, might be all most sufferers must get well. “I do not know why FMT works when it does. I believe there are sufferers who do want the FMT, in any other case they will not get higher. However that could be a a lot smaller pool of individuals than I beforehand would have thought. And I do not know easy methods to determine that individual forward of time,” Rao famous.
Addressing unknown threats
The stool used for FMTs is screened for recognized pathogens, and although it’s attainable for some to slip through the cracks, that is uncommon. The bigger concern transferring ahead is easy methods to take care of unknown pathogen threats.
Take mpox (previously known as monkeypox) for instance. Earlier than the onset of the 2022 global outbreak, the virus was circulating within the inhabitants—and stool banks and clinicians did not learn about it, elevating the danger of transferring mpox to recipients. Relying on the pathogen, such transmission might be dire. “This risk is simply going to be taking place extra typically, and we’re solely going to see extra novel pathogens, not fewer,” Rao warned.
An extra concern: there are hazy associations between the intestine microbiota and quite a few circumstances, from diabetes to cancer. It’s attainable that transferring the microbiota from 1 individual into one other might improve the recipient’s danger for creating these illnesses years down the road. Rao ensures that each affected person for whom he recommends FMT is conscious of this “actual and really believable danger.”
There’s a national registry that goals to trace 4,000 sufferers receiving FMT for 10 years to “determine potential short-term antagonistic outcomes and to seek for long-term security issues.” So, in a decade, clinicians may know extra—however solutions shall be primarily based on epidemiologic associations. It is going to nonetheless be almost unattainable to have a look at the microbial profile of stool and say “I can not give this to affected person X, or they are going to develop situation Y.”
Towards outlined consortia
The “black field” function of FMTs is why these throughout the discipline envision a future the place rationally designed biotherapeutic merchandise with outlined, lab-grown microbial consortia are the norm. Such merchandise can be useful from a security and regulatory standpoint. Nevertheless, reaching that supreme requires taking a reductionist analysis method, teasing aside how particular person microbes (and their merchandise) work together with the host and one another, and the way these interactions is likely to be leveraged for therapeutic use.
This method takes time. But, it’s particularly essential if the objective is to harness microbes to deal with circumstances the place ties to the microbiome will not be as clear and causal as for CDI, and the place FMTs have proven restricted success (e.g., inflammatory bowel disease and irritable bowel syndrome, amongst others).
In Rao’s eyes, the extra nuanced the associations with the intestine microbiota, the extra nuanced the answer must be. “We won’t simply throw stool at individuals [like we did with CDI] and anticipate it to work,” he mentioned. “That is why I am excited that individuals are attempting to develop these outlined consortia as a result of, for different illnesses, that is the one method ahead.”
Quotation:
Fecal microbiota transplants: Previous, current and future (2024, February 9)
retrieved 10 February 2024
from https://medicalxpress.com/information/2024-02-fecal-microbiota-transplants-future.html
This doc is topic to copyright. Other than any honest dealing for the aim of personal examine or analysis, no
half could also be reproduced with out the written permission. The content material is supplied for data functions solely.
[ad_2]
Source link




Discussion about this post