[ad_1]
On the highest, (a) Chemical construction of TPZP. On the underside, (b) Schematic diagram of mixed remedy. Artwork by Michael Rothman. Artwork by Zhaohui Tang. Credit score: Science China Press
Hypoxia-activated prodrugs (HAPs) have the potential to activate particularly in hypoxic tumors and eradicate tumor cells, which has introduced new alternatives for secure and efficient anticancer remedy. Nevertheless, their therapeutic efficacy is restricted by insufficient hypoxia inside the handled tumors.
What’s extra vital, insufficient hypoxia in tumors implies that the distinction in oxygen levels between tumors and regular tissues just isn’t important. The insignificant distinction results in HAPs with a decreased concentrating on capacity to tumors, inadequate activation in tumors, and weakened cytotoxicity to tumor cells, severely impacting anticancer therapy efficacy.
Though hypoxia stratification of sufferers might enhance the therapy efficacy of HAPs, this method would tremendously restrict the scope of HAPs therapy. Like most HAPs, tirapazamine (TPZ) has been permitted for a number of clinical trialscounting on its wonderful anticancer potential. Nevertheless, TPZ didn’t exhibit survival benefits in part III scientific trials as a result of inadequate hypoxia ranges in affected person’s tumors.
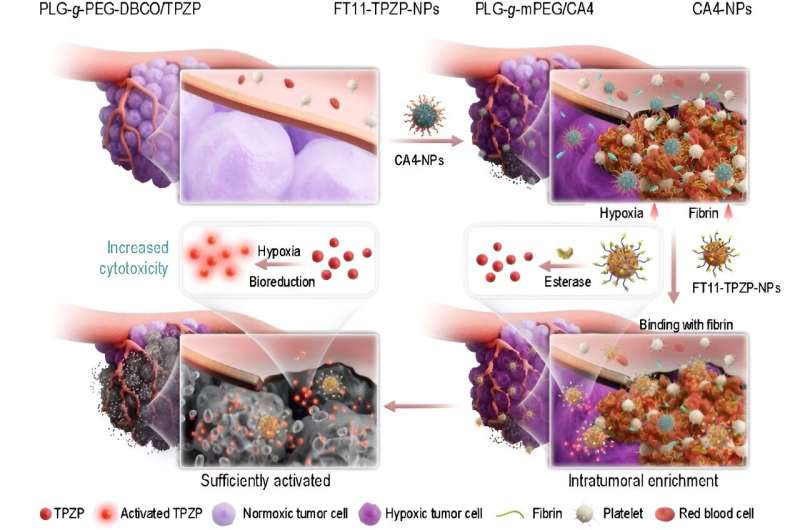
In a brand new analysis article published within the Beijing-based Nationwide Science Evaluationscientists at Changchun Institute of Utilized Chemistry and College of Science and Expertise of China current a brand new common well-rounded nanosystem with amplified energetic concentrating on, enhanced drug activation and elevated hypoxic cytotoxicity to reinforce the anticancer efficacy of TPZ.
It is composed of two indispensable modules: a tumor microenvironment reworking nanodrug (CA4-NPs) and a nanodrug of the cytotoxicity-enhanced TPZ spinoff (FT11-TPZP-NPs). To enhance TPZ’s cytotoxicity, the authors first launched urea to synthesize a collection of urea-containing derivatives of TPZ. All urea-containing TPZ derivatives confirmed elevated hypoxic cytotoxicity (9.51~30.85-fold) in comparison with TPZ whereas sustaining hypoxic selectivity.
TPZP, one among these derivatives, confirmed 20-fold greater cytotoxicity than TPZ whereas sustaining an identical hypoxic cytotoxicity ratio (HCR). To extremely effectively ship TPZP to the tumors and cut back its unwanted effects in regular tissues, the authors additional ready TPZP right into a nano drug with the fibrin-targeting capacity (FT11-TPZP-NPs). CA4-NPs, a vascular disrupting agent, was used to rework the tumor microenvironment with elevated fibrin ranges inside tumors for amplified energetic concentrating on and exacerbate tumor hypoxia for enough drug activation.
By combining with CA4-NPs, FT11-TPZP-NPs can enrich abundantly within the hypoxia-aggravated tumors and activate sufficiently to kill tumor cells. Following a single-dose administration, FT11-TPZP-NPs + CA4-NPs demonstrated a considerable inhibition fee of 98.1% towards CT26 tumor fashions with an preliminary quantity approximating 480 mm3.
Moreover, this therapy resulted within the full eradication of 66.7% of tumors, exhibiting a major antitumor impact. This examine introduces a novel technique for enhancing the anticancer efficacy of TPZ and different HAPs.
Extra info:
Yajun Xu et al, Introducing urea into tirapazamine derivatives to reinforce anticancer remedy, Nationwide Science Evaluation (2024). DOI: 10.1093/nsr/nwae038
Supplied by
Science China Press
Quotation:
Enhancing hypoxia-activated prodrug anticancer remedy (2024, April 3)
retrieved 3 April 2024
from https://medicalxpress.com/information/2024-04-hypoxia-prodrug-anticancer-therapy.html
This doc is topic to copyright. Aside from any truthful dealing for the aim of personal examine or analysis, no
half could also be reproduced with out the written permission. The content material is offered for info functions solely.
[ad_2]
Source link




Discussion about this post