[ad_1]
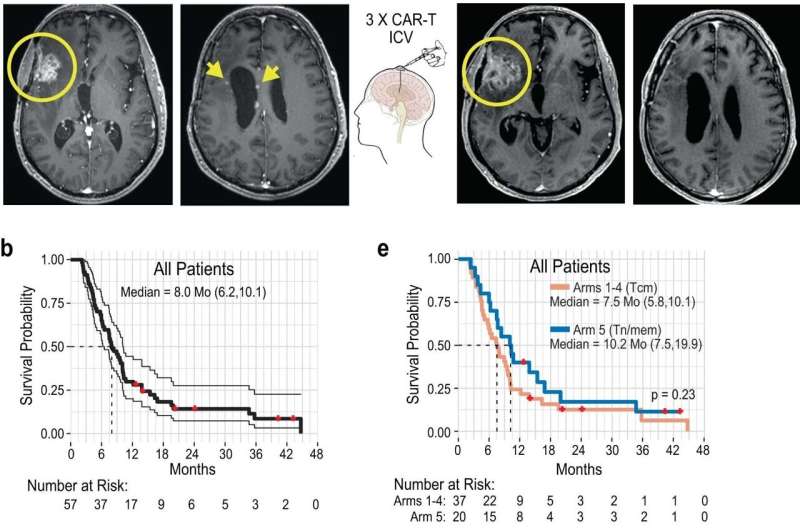
Impact of ICV delivered CAR-T cells for UPN145 and affected person total survival and quality-of-life evaluations. Credit score: Nature Medication (2024). DOI: 10.1038/s41591-024-02875-1
A pioneering Section I CAR T cell remedy trial for the remedy of glioblastoma at Metropolis of Hope demonstrates promising medical exercise towards incurable mind tumors, in accordance with research published in Nature Medication.
The examine, which is the biggest reported trial thus far of CAR T therapy for solid tumorsevaluated CAR T cells engineered to focus on the tumor-associated antigen interleukin-13 receptor alpha 2 (IL13Rα2), a product invented at Metropolis of Hope and completely licensed by Mustang Bio Inc., a Fortress Biotech Inc. Firm.
One of many principal challenges for treating brain cancer is that drugs have issue crossing the blood-brain barrier. To beat that barrier, the trial delivered CAR T cells immediately into the mind tumor and the cerebrospinal fluid, the fluid that protects and surrounds the mind and spinal wire.
Twenty-nine of the 58 sufferers with recurrent high-grade glioma mind tumors, principally glioblastoma, achieved secure illness after remedy with CAR T cells for no less than two months. There have been two partial responses, one full response and a second full response after extra CAR T cell remedy cycles have been delivered below compassionate use. The case was reported in New England Journal of Medication in 2016.
“Glioblastomas are extraordinarily aggressive tumors that depart sufferers with very restricted remedy choices, particularly after they’ve relapsed, however this examine exhibits the potential of CAR T cell remedy in treating mind most cancers,” stated Christine Brown, Ph.D., The Heritage Supplier Community Professor in Immunotherapy and deputy director of the T Cell Therapeutics Analysis Laboratories at Metropolis of Hope, who developed IL13Rα2-targeting CAR T cell remedy.
“This examine can also be essentially the most intensive analysis of delivering CAR T cells on to a mind tumor, which we pioneered at Metropolis of Hope, and units the muse for different research to make the most of this method.”
Members, all of whom had relapsed after prior remedy for GBM with surgical procedure, chemotherapy or radiation, or all of those therapies, obtained intracranial injections of CAR T cells that concentrate on IL13Rα2, which is overexpressed in most glioblastomas.
Doses of the remedy have been escalated because the trial progressed and all doses examined have been well-tolerated. Three routes of administration have been evaluated: direct injection to the tumor website, infusion into the cerebrospinal fluid or injection into each areas.
The median overall survival for all sufferers was eight months. The trial culminated in treating a affected person cohort that used an optimized manufacturing course of and injected CAR T cells at each the tumor website and into the cerebrospinal fluid.
For this ultimate affected person cohort, researchers have been capable of set up a maximal possible dose and located that these sufferers had the perfect median total survival of 10.2 months, which was increased than the anticipated survival price of six months in sufferers with recurrent glioblastoma.
“These have been closely pretreated sufferers so we weren’t positive how they’d do with CAR T cell remedy,” stated Behnam Badie, M.D., The Heritage Supplier Community Professor in Gene Remedy, chief of neurosurgery at Metropolis of Hope and the examine’s senior writer. “However a few of them even did higher than how they initially responded to plain of care remedies.”
Peter Valadez, 58, of Ontario, California, is likely one of the sufferers who achieved full remission. A former promoting government and father of three, he was recognized with a high-grade glioma in 2014. The tumor on the fitting facet of his mind was surgically eliminated 3 times. After his final surgical procedure, non-Metropolis of Hope medical doctors instructed him the tumor couldn’t be eliminated once more.
“One physician instructed me to get my affairs so as as a result of I solely had just a few months to stay,” Valadez stated. “She additionally instructed me to look into receiving CAR T cell remedy at Metropolis of Hope. I did, and that’s the reason I’m nonetheless right here right this moment.”
Valadez obtained the CAR T cell remedy in July 2018 with minimal negative effects. He did expertise wound-related issues, possible the results of having a number of surgical procedures and prior radiation remedy to deal with his tumor. The left facet of his physique is partially paralyzed.
The daddy of three grown youngsters and a 5-year-old granddaughter has not obtained another therapies for the reason that CAR T remedy over 5 and a half years in the past.
Along with evaluating the security and feasibility of IL13Rα2 CAR T cells delivered regionally for this trial, Brown, Badie and the workforce additionally needed to be taught extra about which sufferers would possibly reply greatest to the remedy. By sampling tumors and cerebrospinal fluid on the supply websites, they discovered sure markers that have been positively related to survival and with CAR T cell administration and bioactivity, respectively.
“The thought you could ship a remedy and pattern the setting on the similar time to research modifications within the mind could be very distinctive and will have a big impact on the sphere,” stated Badie. “I feel individuals will likely be adopting these applied sciences that may assist us acknowledge potential mechanisms of resistance to or failure of remedy.”
The trial outcomes construct on greater than a decade of collaborative work between Brown, Badie, Stephen J. Forman, M.D., director of the T Cell Therapeutics Analysis Laboratories, and different Metropolis of Hope researchers on IL13Rα2-CAR T cells.
“This was an in-house translation of scientific findings to human trials—a transparent instance of how one can take a look at a novel remedy in sufferers, go to the lab to enhance it after which return to the clinic with a more practical remedy,” stated Badie.
“I feel that forwards and backwards is de facto distinctive to Metropolis of Hope and is simply potential as a result of unbelievable collaboration between our completely different groups and the potential Metropolis of Hope has to provide the CAR T cells on-site.”
Metropolis of Hope, with its medical, analysis and manufacturing amenities all on one campus, is uniquely positioned to harness the “bench to bedside” sources vital for the invention, improvement, manufacturing, high quality assurance, testing and deployment of modern remedies.
Subsequent, the workforce says that future randomized research in bigger affected person cohorts will likely be wanted to substantiate and develop their findings associated to the essential parameters for profitable CAR T remedy.
Brown says they’re additionally excited to begin a trial to engineer CAR T cells to be resistant to remodeling development factor-beta, a dominant tumor suppressor, in addition to a examine to mix completely different CARs that may goal a number of antigens and construct new bispecific CARs that would have interaction two illness targets as a substitute of only one.
“Between the optimistic outcomes we noticed in our Section I examine and the brand new avenues we’re exploring, we hope our work can have a dramatic influence on the sphere of immunotherapy and the lives of cancers sufferers all over the world,” she stated.
The examine workforce additionally included researchers from Translational Genomics Analysis Institute in Phoenix, which is a part of Metropolis of Hope.
Extra info:
Christine E. Brown et al, Locoregional supply of IL-13Rα2-targeting CAR-T cells in recurrent high-grade glioma: a section 1 trial, Nature Medication (2024). DOI: 10.1038/s41591-024-02875-1
Supplied by
City of Hope National Medical Center
Quotation:
Chimeric antigen receptor (CAR) T cell remedy exhibits medical exercise in sufferers with aggressive mind tumors (2024, March 7)
retrieved 8 March 2024
from https://medicalxpress.com/information/2024-03-chimeric-antigen-receptor-car-cell.html
This doc is topic to copyright. Other than any honest dealing for the aim of personal examine or analysis, no
half could also be reproduced with out the written permission. The content material is supplied for info functions solely.
[ad_2]
Source link




Discussion about this post