[ad_1]
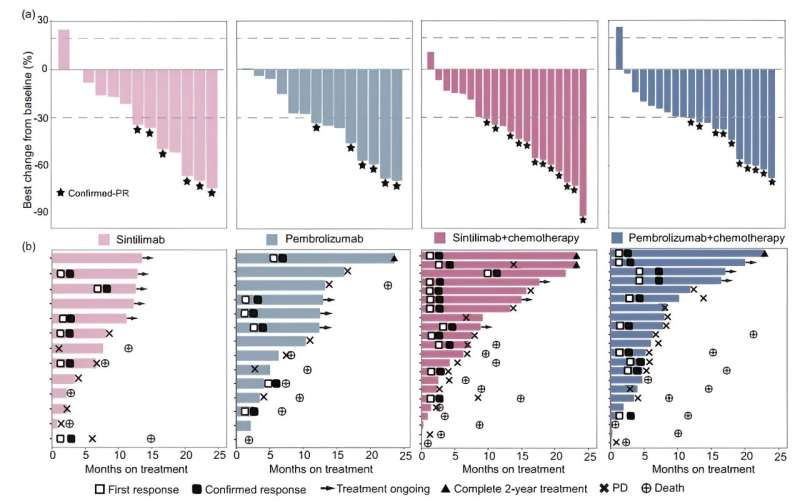
The most effective change in goal lesion measurement and remedy length for all sufferers are illustrated in waterfall and swimmer plots. Credit score: Science China Press
A examine published within the journal Science Bulletin was led by Yi-Lengthy Wu (Guangdong Lung Most cancers Institute, Guangdong Provincial Individuals’s Hospital, Chinese language Thoracic Oncology Group (CTONG)).
On this open-label, part 2 examine (NCT04252365), sufferers with superior NSCLC with out EGFR or ALK alterations have been randomized (1:1) to obtain sintilimab or pembrolizumab monotherapy (PD-L1 TPS ≥50%), or sintilimab or pembrolizumab plus platinum-based chemotherapy (PD-L1 TPS <50%). The pattern measurement was calculated by optimum two-stage design. The first endpoint was the target response price (ORR).
The examine included 71 sufferers (sintilimab arms, n=35; pembrolizumab arms, n=36) and met its main endpoint, with a confirmed ORR of 51.4% (18/35) within the sintilimab arms. The confirmed ORR (95% confidence interval) was 46.2% (19.2, 74.9) and 42.9% (17.7, 71.1) for sufferers handled with sintilimab and pembrolizumab monotherapy, and 54.5% (32.2, 75.6) and 45.5% (24.4, 67.8) for these handled with sintilimab- and pembrolizumab-based mixture therapies.
The median progression-free survival was 6.9 versus 8.1 months for all sintilimab-treated versus all pembrolizumab-treated sufferers, respectively, wherein it was 7.6 versus 11.0 months in monotherapy and seven.4 versus 7.1 months together therapies.
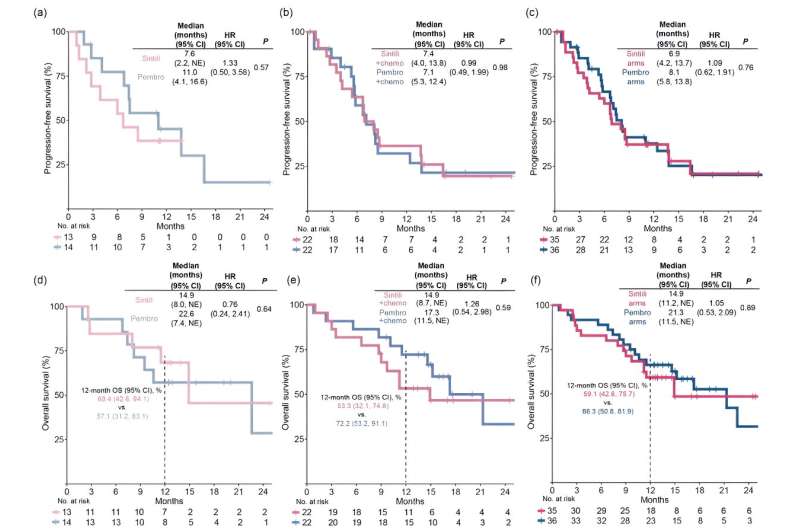
Kaplan-Meier estimates of progression-free survival assessed by RECIST v1.1 per investigator in sufferers handled with (a) sintilimab versus pembrolizumab as monotherapy, (b) sintilimab versus pembrolizumab plus platinum-based doublet chemotherapy, respectively, as mixture remedy, and (c) sintilimab versus pembrolizumab arms together with sufferers who obtained mono- and mixture remedy. Kaplan-Meier estimates of total survival in monotherapy (d), mixture remedy (e), and sintilimab versus pembrolizumab arms (f). Credit score: Science China Press
The median total survival was 14.9 versus 21.3 months for all sintilimab-treated versus all pembrolizumab-treated sufferers, respectively, wherein it was 14.9 versus 22.6 months in monotherapy and 14.7 versus 17.3 months together therapies. Remedy-related opposed occasions have been in line with the protection outcomes of monotherapy and mixture remedy in earlier part III research. Nonetheless, the incidence of rash was larger with sintilimab than with pembrolizumab monotherapy.
That is the primary potential part 2 examine to immediately examine two anti-PD-1 antibodies as first-line remedy in superior NSCLC. Sintilimab was efficacious and well-tolerated no matter PD-L1 expression stage in sufferers with superior NSCLC and had comparable efficacy and security to pembrolizumab.
Extra data:
Si-Yang Maggie Liu et al, PD-L1 expression steering on sintilimab versus pembrolizumab with or with out platinum-doublet chemotherapy in untreated sufferers with superior non-small cell lung most cancers (CTONG1901): A part 2, randomized, managed trial, Science Bulletin (2023). DOI: 10.1016/j.scib.2023.12.046
Supplied by
Science China Press
Quotation:
PD-L1 expression steering on sintilimab vs. pembrolizumab with/with out chemotherapy in untreated sufferers (2024, March 5)
retrieved 5 March 2024
from https://medicalxpress.com/information/2024-03-pd-l1-guidance-sintilimab-pembrolizumab.html
This doc is topic to copyright. Other than any truthful dealing for the aim of personal examine or analysis, no
half could also be reproduced with out the written permission. The content material is offered for data functions solely.
[ad_2]
Source link




Discussion about this post