[ad_1]
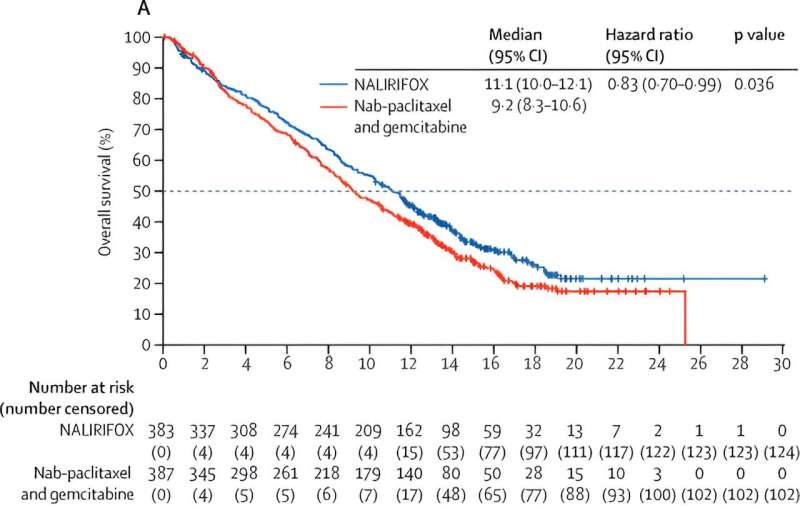
Kaplan–Meier estimates of general survival (A) and progression-free survival (B) NALIRIFOX=liposomal irinotecan together with fluorouracil, leucovorin, and oxaliplatin. Credit score: The Lancet (2023). DOI: 10.1016/S0140-6736(23)01366-1
A four-drug chemotherapy routine of irinotecan liposome (Onivyde) together with oxaliplatin, leucovorin, and fluorouracil—collectively known as NALIRIFOX—has been accredited by the U.S. Meals and Drug Administration (FDA) for the first-line therapy of metastatic pancreatic adenocarcinoma.
The FDA approval was primarily based on outcomes of the NAPOLI 3 trial, a research led by Dr. Zev Wainberg, co-director of the UCLA Well being GI Oncology Program and a researcher on the UCLA Well being Jonsson Complete Most cancers Middle.
Findings from the NAPOLI 3 trial have been first introduced on the 2023 American Society of Medical Oncology Gastrointestinal Cancers Symposium annual assembly and published in The Lancet in September of 2023. Wainberg, the worldwide principal investigator for the trial, reported NALIRIFOX resulted in longer general survival than a two-drug protocol comprised of nab-paclitaxel (Abraxane) and gemcitabine.
“The FDA approval is important due to how troublesome it’s to deal with metastatic pancreatic most cancers,” stated Wainberg, who can be a professor of drugs on the David Geffen Faculty of Medication at UCLA. “Metastatic pancreas most cancers has lengthy been acknowledged as a really troublesome sort of most cancers to deal with, however this research represents a doable new benchmark commonplace for present therapies and a promising avenue for ongoing analysis and drug improvement.”
The part 3 research included 770 sufferers with pancreatic ductal adenocarcinoma, which makes up 95% of pancreatic cancers. Members have been from 250 websites in 25 nations and have been randomly assigned to NALIRIFOX or the two-drug remedy.
Sufferers within the NALIRIFOX group had an general survival of 11.1 months, in contrast with 9.2 months for these within the two-drug arm. Development-free survival additionally elevated with NALIRIFOX to 7.4 months versus 5.6 months with the two-drug routine, which interprets right into a 30% discount within the danger of illness development or demise.
The research is believed to be the primary metastatic pancreatic most cancers research in almost a decade to have a optimistic endpoint for general survival.
Most instances of pancreatic most cancers are recognized at extra superior levels when the illness is extra aggressive and has already began spreading to different components of the physique. There are additionally restricted therapy choices, which contributes to the excessive fatality fee of pancreatic most cancers. Solely about 13% of sufferers survive 5 or extra years. In 2024 alone, the American Most cancers Society estimates that round 35,000 individuals are anticipated to die from the illness.
The commonest unwanted side effects individuals skilled within the trial included diarrhea, fatigue, nausea, vomiting, lowered urge for food, belly ache, mucosal irritation, constipation and decreased weight.
Extra info:
Zev A Wainberg et al, NALIRIFOX versus nab-paclitaxel and gemcitabine in treatment-naive sufferers with metastatic pancreatic ductal adenocarcinoma (NAPOLI 3): a randomised, open-label, part 3 trial, The Lancet (2023). DOI: 10.1016/S0140-6736(23)01366-1
Supplied by
University of California, Los Angeles
Quotation:
FDA approval of 4-drug mixture for front-line therapy of metastatic pancreatic most cancers (2024, February 16)
retrieved 17 February 2024
from https://medicalxpress.com/information/2024-02-fda-onivyde-combination-metastatic-pancreatic.html
This doc is topic to copyright. Aside from any truthful dealing for the aim of personal research or analysis, no
half could also be reproduced with out the written permission. The content material is offered for info functions solely.
[ad_2]
Source link




Discussion about this post