[ad_1]
Cleveland Clinic researchers lately published a examine in Cell Reviews that shines new mild on a next-generation goal of immunotherapy—the immune checkpoint protein VISTA.
Li Lily Wang, Ph.D., and a analysis group discovered a brand new, exploitable pathway for VISTA, which researchers know helps tumors develop “regulatory immune cells” known as myeloid-derived suppressor cells (MDSCs). MDSCs stop different immune cells that assault tumors, like T-cells, from functioning. Since VISTA’s discovery in 2011, researchers have puzzled precisely how the protein promotes MDSC improvement.
The speculation was that the regulatory operate of VISTA was associated to its capability to instantly work together with T-cells and reduce their capability to combat illnesses like an infection and most cancers. Dr. Wang’s examine proved that VISTA—and subsequently immunotherapy resistance—operates by means of a very completely different pathway.
Immunotherapies prepare the immune system to combat most cancers, however proteins like VISTA can get in the best way. Co-first creator Amin Zakeri, Ph.D., explains that VISTA is a crucial “immune checkpoint protein” that poses a serious impediment to immunotherapies as a result of it prevents our immune cells from correctly responding to therapy.
Below wholesome circumstances, VISTA retains immune techniques in verify and prevents assaults on wholesome cells. Throughout most cancers, nevertheless, VISTA can do its job too properly and prevents the physique from efficiently preventing off a tumor.
“Immunotherapies permit physicians to deal with and handle cancers that would not beforehand be handled by chemotherapy or radiation therapy with a 20% to 30% price of success,” says Dr. Wang of Cleveland Clinic’s Division of Translational Hematology & Oncology Analysis. “That is an unbelievable development, however to assist the 70-80% of sufferers who fail to reply to this therapy, we should perceive how tumors can evade the immune system.”
The investigation into VISTA and MDSCs was led by three co-first authors: Dr. Zakeri, Keman Zhang, Ph.D., and Tyler Alban, Ph.D. The group confirmed that blocking VISTA in preclinical fashions diminished tumor dimension and elevated the flexibility to reply to immunotherapy. They then took their examine a step additional to be taught precisely how this response may happen.
“VISTA is concerned in a number of pathways and works in a couple of other ways, so we anticipated to realize extra perception into the already identified pathways with our mechanistic research,” says Dr. Zhang, a analysis affiliate in Dr. Wang’s lab. “Think about our shock after we discovered a very new mechanism altogether.”
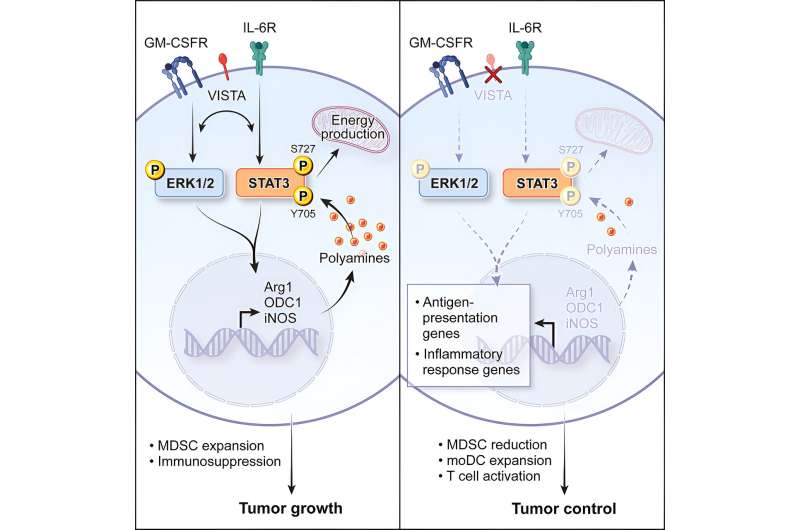
Within the newly found pathway, VISTA is a part of a suggestions loop that promotes the activation of a gene known as STAT3 (which encodes a protein of the identical title) and the manufacturing of metabolites known as polyamines. Polyamines assist cells to develop, operate, and replicate, however throughout most cancers, they will accumulate and induce tumor-associated MDSC improvement, which helps the tumor resist immunotherapy.
There was a spotlight within the biomedical analysis neighborhood on utilizing STAT3 inhibitors as polyamine-blocking therapeutics, with restricted success.
“STAT3 is a really engaging drug goal for immunotherapy, however to this point, the potential drug candidates that inhibit it have largely did not make an influence because of toxicity,” says Dr. Wang. “Our knowledge raises the potential of influencing STAT3 and polyamine manufacturing not directly by concentrating on VISTA as a substitute.”
Toxicity in different tissues is not as a lot of a priority as a result of the VISTA protein is disproportionately enriched in tumor-associated MDSCs, Dr. Wang added.
These implications are particularly thrilling as a result of STAT3 has already been extensively studied, says Dr. Alban. Dr. Alban works as a postdoctoral fellow at Cleveland Clinic’s Middle for Immunotherapy & Precision Immuno-Oncology. Connecting VISTA with a “identified agent” like STAT3 will make follow-up research and drug improvement a lot simpler, Dr. Alban says, as a result of scientists’ skills to make therapeutics are restricted by their data of the molecule they want to goal.
Analysis is presently underway to leverage the connection between STAT3 and VISTA in pursuit of an efficient drug candidate. Dr. Wang says her group’s examine has a excessive translational influence on a number of human cancers, already tying VISTA expression with decrease survival outcomes in endometrial most cancers.
The group is creating the strategies wanted to find out what different most cancers sorts use this mechanism in people.
“There are lots of new collaborations and initiatives within the work to discover the mechanisms behind VISTA and its contribution to the tumor microenvironment,” says Dr. Zakeri. “VISTA has opened lots of new home windows for immune and most cancers analysis.”
Extra data:
Keman Zhang et al, VISTA promotes the metabolism and differentiation of myeloid-derived suppressor cells by STAT3 and polyamine-dependent mechanisms, Cell Reviews (2024). DOI: 10.1016/j.celrep.2023.113661
Quotation:
Analysis reveals a course of tumors use to induce immune suppressor cells and evade immunotherapy (2024, February 9)
retrieved 9 February 2024
from https://medicalxpress.com/information/2024-02-reveals-tumors-immune-suppressor-cells.html
This doc is topic to copyright. Aside from any truthful dealing for the aim of personal examine or analysis, no
half could also be reproduced with out the written permission. The content material is offered for data functions solely.
[ad_2]
Source link




Discussion about this post