[ad_1]
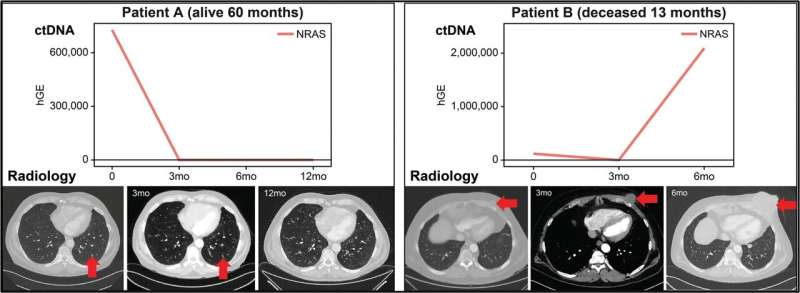
Circulating tumor DNA (ctDNA) correlates with radiology illness evaluation and affected person final result. For affected person A, biomarkers of their blood decreased over time and the radiology confirmed the disappearing tumor over time. This affected person had medical final result and was alive at 60 months after prognosis. Affected person B sadly confirmed rising ranges of biomarkers of their ctDNA over time und succumbed to their illness at 13 months Credit score: The Journal of Molecular Diagnostics
Circulating tumor DNA (ctDNA) is rising as a blood-based biomarker for a lot of strong tumor sorts, together with melanoma. A brand new study that assessed ctDNA within the blood of sufferers with BRAF wild-type (BRAF WT) stage III and IV melanoma concludes that measuring ctDNA could result in different therapy choices and higher outcomes for these sufferers, report investigators in The Journal of Molecular Diagnostics.
For sufferers with BRAF WT stage III and IV melanoma, there’s an pressing medical must establish prognostic biomarkers and biomarkers to foretell therapy response. Roughly 40% to 50% of all sufferers with late-stage cutaneous melanoma have a BRAF p.V600 mutation; in these sufferers therapy with selective BRAF and MEK inhibitors (BRAFi/MEKi) considerably prolongs each progression-free survival and general survival.
Immune checkpoint inhibitor (ICI) remedy exhibits promising outcomes when mixed with focused therapies in sufferers with BRAF p.V600-positive tumors. Longer general survival is noticed when ICI therapies are mixed (anti-PD-1 and anti-CTLA-4). Nevertheless, BRAF WT sufferers or sufferers harboring an alternate BRAF variant don’t profit from these combinatorial ICI therapies.
Lead investigators Vanessa F. Bonazzi, Ph.D., and Lauren G. Aoude, Ph.D., The Frazer Institute, The College of Queensland, clarify, “Present medical apply means each BRAF WT and BRAF p.V600 optimistic sufferers obtain ICI remedy. Nevertheless, they don’t all profit. We urgently must establish biomarkers predictive of illness recurrence and response to different therapies after ICI failure. Sufferers might be higher matched to particular therapies that focus on genomic alterations of their tumors utilizing biomarkers that establish particular tumor targets and information affected person administration. The intention of this research was to establish targetable variants current within the blood that will result in different therapy choices and higher outcomes for BRAF WT sufferers.”
Abnormalities in ctDNA shed into the bloodstream by lifeless or dying tumor cells could be detected and measured in noninvasive exams. Investigators assessed ctDNA from the plasma samples of 106 sufferers. Additionally they included serial blood collections for a subset of 16 sufferers to carry out a longitudinal study. Variants had been detected in 85% of sufferers, all in targetable pathways.
Apparently, in melanoma, the focus of ctDNA had prognostic worth. Sufferers with stage IV melanoma with low ctDNA concentrations, lower than 10 ng/mL, had considerably higher disease-specific survival and progression-free survival. Sufferers with each a excessive focus of ctDNA and any detectable ctDNA variants had the worst prognosis. As well as, these outcomes indicated that longitudinal modifications in ctDNA correlated with therapy response and illness development decided by radiology.
Dr. Bonazzi notes, “Our outcomes confirmed that modifications in ctDNA over time correlated with response to therapy and development of the illness as assessed by radiology. Measuring sufferers’ ctDNA all through their therapy would assist comply with their potential response and adapt the therapy routine accordingly.
“Whereas sufferers can have a PET/CT scan each six months, a blood test might be carried out month-to-month and supply an correct reply adopted by fast actions. If for instance, the affected person ctDNA profile exhibits a PIK3CA mutation from baseline, you may comply with it in numerous blood samples from this affected person over time, and down the observe, you may use a PIK3CA inhibitor and hopefully be capable of stop potential recurrence of illness.”
Dr. Aoude provides, “The sequencing of the ctDNA gives data for potential drug targets. Moreover, the quantification might present further data relating to disease-specific survival and progression-free survival.”
This research confirms that ctDNA might be used as a noninvasive liquid biopsy to establish recurrent illness and detect targetable variants in sufferers with late-stage melanoma.
The investigators conclude that the power to make use of ctDNA as a medical software is extremely engaging as a result of it’s a noninvasive, repeatable take a look at that may supply further medical data relating to therapy response and tumor recurrence. This research demonstrates the worth of ctDNA in sufferers with stage IV melanoma as a software to detect somatic variants with clinically targetable mutations, as a prognostic indicator in sufferers with stage IV melanomaand as a software for monitoring therapy response to ICI remedy.
Extra data:
Lauren G. Aoude et al, Circulating Tumor DNA, The Journal of Molecular Diagnostics (2023). DOI: 10.1016/j.jmoldx.2023.06.014
Quotation:
Blood-based biomarker could redefine future therapy for superior melanoma (2023, October 4)
retrieved 4 October 2023
from https://medicalxpress.com/information/2023-10-blood-based-biomarker-redefine-future-treatment.html
This doc is topic to copyright. Aside from any honest dealing for the aim of personal research or analysis, no
half could also be reproduced with out the written permission. The content material is offered for data functions solely.
[ad_2]
Source link




Discussion about this post