[ad_1]
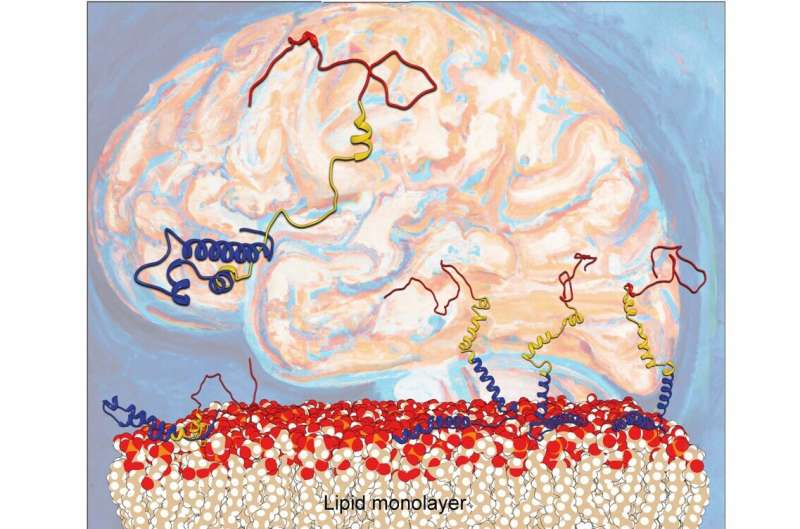
For a number of years, scientists have identified that Parkinson’s illness is expounded to misfolding of the protein alpha-synuclein. Deposited aggregates of a protein known as alpha-synuclein (α-syn) can harm and kill nerve cells, resulting in neurological dysfunction. It has been identified that lipid layers on cell surfaces can speed up the misfolding course of; nonetheless, the microscopic mechanisms concerned have been a thriller.
Now, scientists from the Departments of Chemistry and iNANO at Aarhus College have unraveled what really occurs when alpha-synuclein binds to lipid layers. A analysis workforce headed by Steven Roeters and Tobias Weidner reveals how the lipid floor influences the orientation and folding of α-syn.
The Aarhus researchers report in Nature Communications that the orientation of α-syn adjustments at elevated protein concentrations, going from the beforehand reported flat geometry to a hitherto unknown upright conformation. Because of this, the α-syn molecules can refold into harmful aggregates extra simply.
Understanding α-syn folding is of nice significance to medical researchers from many alternative views. Though neurodegenerative diseases are a quickly rising downside in our getting old societies, they nonetheless can’t be cured. The refolding of proteins and their interactions with cell surfaces is a much-debated matter in present Parkinson’s analysis.
To know how α-syn behaves at surfaces, the researchers produced and used artificial proteins, putting them in a single layer on a mannequin nerve cell floor. To analyze the binding, motions and folding of this nanometer-thin layer of proteins, the workforce used a laser-based spectroscopic technique known as sum frequency era spectroscopy. The complicated datasets had been disentangled utilizing a computational technique particularly developed with the division’s pc simulations group.
Thanks to those new findings, it could now be potential to observe the misfolding course of and the working mechanisms of potential medical medicine in molecular element. “Sooner or later we’d use this technique to display screen for efficient therapies to forestall the illness,” says Steven Roeters, who’s now a analysis affiliate at Amsterdam UMC.
Inspired by the constructive outcomes, the Aarhus analysis teams need to lengthen their investigations to different surfaces which α-syn may encounter and which replicate the way in which our society impacts our environment, e.g. plastic contaminants.
“We plan to look at the interactions of α-syn with artificial materials, corresponding to microplastics to know the impression of environmental situations on Parkinson’s,” explains Affiliate Professor Tobias Weidner, whose working group at Aarhus College specializes within the characterization of proteins at floor and nanomaterials.
Extra data:
Steven J. Roeters et al, Elevated concentrations trigger upright alpha-synuclein conformation at lipid interfaces, Nature Communications (2023). DOI: 10.1038/s41467-023-39843-1
Quotation:
Discovery of protein orientation helps scientists perceive Parkinson’s illness (2023, September 20)
retrieved 20 September 2023
from https://medicalxpress.com/information/2023-09-discovery-protein-scientists-parkinson-disease.html
This doc is topic to copyright. Other than any honest dealing for the aim of personal examine or analysis, no
half could also be reproduced with out the written permission. The content material is supplied for data functions solely.
[ad_2]
Source link




Discussion about this post